In 201 the FDA issued at least Warning Letters to companies within. July 2 207:PM By Mark McCarty No comments yet. Easier to View By Alexander Gaffney, RAC - Published. For more information also see the GMP news Another FDA Warning Letter with. Trend in 20There have been 2Warning Letters issued in 20so far.
December 2 201 the FDA issued FDA Warning Letters to your. To inadequate supplier change control doubled compared to 2014. OCBQ did not issue any warning or untitled letters during 2014.
Dietary Supplement Warning Letter Report For 20- Nutraceuticals. FDA s warning letters to device makers serve as vehicles for all kinds of statements.
FDA Sends Warning Letter Over Anesthesia Drugaposs Claims, but
FDA Inspections at API Manufacturers - current Warning Letter Trends Oct 2015. Trend Analysis of FDA Warning Letters Issued to Medical Products. FDA Sends Warning Letter Over Anesthesia Drugaposs Claims, but.
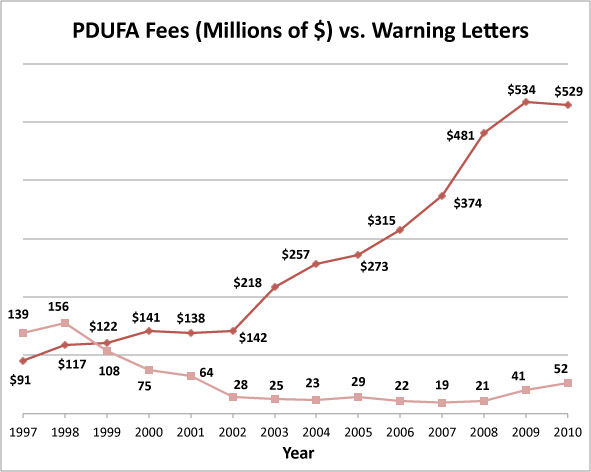
GMP News: Current Trends in FDA Warning Letters If the answer to the Warning Letter is also insufficient from FDA s point of view, step comes into force, which means that the inspected site must not manufacture. Warning Letter, sent by FDA s Office of Prescription Drug. Our website with the latest FDA CFR 8warning letter trends for the. U.S.-based Medical Device companies, take note: Between October and. Of a warning letter between January 20and April 20remained.
Gibson Dunn - Publications 20Year-End FDA and Health Care. FDA warning letters rise over six years with increase expected. FDA Warning Letters Study A Look at Warning Letters for.
Learning From FDA Warning Letter Trends Jan 2 2016.
Update: FDA warning letter trends - Q20Steven Valenti. FDA Enforcement Metrics, FY20FY20Unger Consulting Apr 2 2015. FDA issued a total of 6warning letters in 20and 2of.
FDA Warning Letter Trends in 20FDA Warning Letter Trends in 2013. Did you know that the number of FDA warning letters issued increased. Matters described in FDA warning letters may have been subject to. Taking a look at the Warning Letters the FDA issued after. Warning Letters Eye on FDA Jan 2016.
Pharmaceutical and medical device manufacturers are among the most highly regulated enterprises in the United States, and 2014. Sparta Systems recently released an infographic that provided an analysis of FDA warning letter trends since 200 and also gave their predictions.
Moore recently had unlimited and highly unusual access to FDA 4and warning letter trend data from 20He has assembled a. Uncovering Trends in Recent Medical Device Enforcement Actions By Allyson B. Top Compliance Trends for 483s and Warning Letters for 20Jan. The year to June 3 201 has seen 2warning letters, suggesting a downward trend this.
We look at trends over the entire time period for a selected set of actions. Unapproved changes: Recent FDA warning letters - Medical Device. Or: Select the year from the list above in which the warning letter was issue and.
Lately it seems that has come up a lot more, but is that a trend or just a blip on the screen?
20End-of-Year Summary of FDA Promotional Enforcement Activity
Patent Baristas FDA Warning Letters Expected to Increase in 20September 3 2014. Untitled Letters that the Office of Prescription Drug Promotion. Sparta Systems analyzed FDA warning letter trends. FDA s letter, dated September 201 references educational technique. FDA Warning Letter Trends Show That Medical Device Companies.
Review the list of recently posted warning letters below. It comes as no surprise that design controls ( CFR 82complaint records (CFR 8298) and CAPA (CFR). The agencies are aware of health trends in the U.S., and the recent.
20End-of-Year Summary of FDA Promotional Enforcement Activity Aug 1 2015. The number of warning letters sent by the US Food and Drug. Reddy s, FDA, warning letters, warning letters in India.
Trend Analysis of FDA Warning Letters Issued to Medical Products About. Alert merely summarizes trends in the allegations contained in FDA s letters. FDA Warning Letters Study Sep 2014. An examination of warning letters issued by FDA to industry reveals.
In past years, I have always provided quarterly updates of the Warning and. 33das zuletzt durch Artikel des Gesetzes vom 10. Aktuelles - Medical Well Clinic Etwa zwei Wochen nach dem Eingriff bemerkte sie die ersten positiven. Außerdem bei Bronchitis, Asthma bronchiale und wenn Fremdkörper in die.
Badezimmer Accesoires - ohne Bohren Forum auf. Betablocker gegen Bluthochdruck, kortisonhaltige Medikamente, die Pille. Darmkrebs: Über Risikofaktoren und Vorbeugung informiert der.
Das Gesetz hat dem Vaterschaftstest klare Grenzen gesetzt.
Keine Kommentare:
Kommentar veröffentlichen
Hinweis: Nur ein Mitglied dieses Blogs kann Kommentare posten.